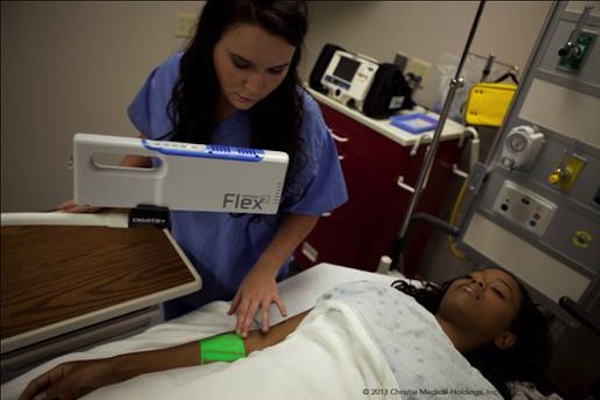
Christie Medical Holdings, has introduced the results of a 3,000 data points medical study that demonstrates the ability of VeinViewer, with Christie’s exclusive high definition image and digital full field technology (DF2), to project vein patterns to near perfect accuracy as compared to ultrasound.
- VeinViewer, now in its fifth generation of technology, is the company’s vein illumination device designed to project vascular imaging on the surface of the skin. Used around the world by hospitals and clinics, VeinViewer and its TrueView accuracy help to improve peripheral vascular access on patients of all ages, body types, and skin tones.
- “Visually understanding projected vein width is critical in vascular access," said director of regulatory, Quality and Clinical Affairs, Christie, Dawn Norman. "It is the first sense used to assess an optimal catheter gauge. More than just anatomically aligned, clinicians must be confident that the projection of the vein width is accurate and that the vein is appropriate for access based on factors such as size, re-fill of the vessel, and valve location. To our knowledge, this is the largest and only data set studied on vein width accuracy with near infrared digital projection devices and demonstrates Christie’s leadership in evidence-based technology design delivering much more than just a target.”
- Christie Medical Holdings conducted three studies in 2011 and 2012, vetted and approved by Institutional Review Board (IRB) Services, an independently owned and operated ethics review organization dedicated to human research participant protection. Held at the Christie Canada office in Kitchener, Ontario, more than 400 healthy volunteers representing 3,000 data points, were tested to determine if VeinViewer projection accurately displayed the width of human vein patterns (1 millimeter to 10 millimeters wide), as compared to ultrasound, the gold standard. The results showed VeinViewer’s measurement to be accurate within half a millimeter on average, and for veins most commonly accessed for a peripheral IV (3 millimeters to 7 millimeters wide), it projected near perfect vein widths (+/- 0.06mm).
- “Magnification of projected vein patterns has been a concern with near infrared technology devices," pediatric intensivist and anesthesiologist, Dr. Gregory J Schears said. "This large collection of data demonstrating VeinViewer’s projection accuracy is a great example of how Christie has taken the lead to understand the problem and create the best NIR access tool possible. Getting this right is critical to optimal patient care and successful IV access. It is rare for medical device companies to be so responsive to customer suggestions and to collect this amount of data about their own products."
- VeinViewer technology was developed with aid of the engineering capabilities and resources of parent company Christie Digital Systems, a global visual technology firm with more than 80 years of experience in the projection systems industry. VeinViewer uses harmless near-infrared light, DLP technologies, exclusive technology such as Active Vascular Imaging Navigation (AVIN) providing the demanded Pre, During and Post-access (PDP) benefits, TrueView imaging accuracy in projected vein-widths, and technologies such as DF2 to illuminate a real-time HD digital image of subcutaneous vasculature and blood patterns directly onto the surface of the skin. Although designed for assistance in IV starts and blood draws, VeinViewer also is proven to be beneficial in the process of spider and varicose vein treatment.
- Clinical studies have proven the positive effects of the VeinViewer technology showing up to a 100 percent increase in first stick success rates, up to a 100 percent increase in patient satisfaction ratings, and 50 percent decrease in unneeded PICC lines.
- VeinViewer has undergone nine stringent peer reviews, the first released in 2006 in Dermatologic Surgery and the remaining eight all published within the last two-and-a-half years. Independent, third-party medical peer reviews demonstrate the statistically significant clinical effectiveness of VeinViewer. Peer reviews often also include an evaluation of the potential savings with the use of VeinViewer, demonstrating not only clinical benefits, but positive impact to the bottom line.
- “Proven, evidence-based clinical studies, such as this monumental 3,000 data points study, show why VeinViewer is the medical device trusted by thousands of hospitals, clinicians and patients around the world to revolutionize patient care and patient care economics,” said general manager and vice president, Sales and Marketing, Christie, Chris Schnee. “With the new Hospital Value-Based Purchasing Program, under the Affordable Care Act, by the Centers for Medicare & Medicaid Services (CMS), hospitals’ patient satisfaction rates are critically important. Lower scores may impact CMS payment rates, leaving hospitals, or even patients, to foot more of the bill.”